Background. Targeting phosphatidylinositol 3-kinase (PI3K) has emerged as an efficacious approach for treatment of chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL). However, toxicities of the PI3K inhibitors are an important concern limiting their use. Duvelisib is a potent oral inhibitor of both the PI3K-δ and PI3K-γ isoforms with proven efficacy in CLL/SLL. Here we report the results of a phase 2 study evaluating efficacy and safety of duvelisib with intermittent dosing in patients with relapsed/refractory CLL/SLL
Methods. This open-label, single arm, phase 2 study enrolled patients with CLL/SLL treated with at least 1 prior line of therapy with active disease as defined by the iWCLL 2008 criteria. Duvelisib was given at a dose of 25 mg PO BID for 28-day cycles for a total of 12 weeks during the induction phase, followed by 75 mg PO BID 2 days on and 5 days off for repeated 28-day cycles during the maintenance phase until death, intolerance or disease progression. The primary endpoint was progression free survival (PFS) at 12 months; secondary endpoints were safety and clinical benefit. The trial was originally planned to enroll 27 patients but was stopped early due to difficulties with enrollment following changing FDA guidance for the use of PI3K inhibitors.
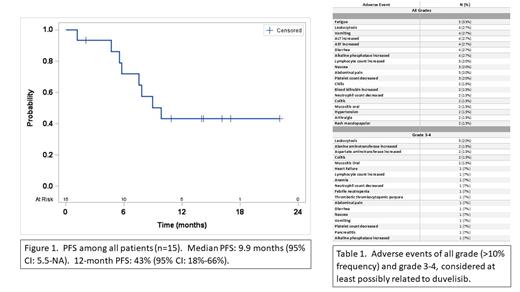
Results. Fifteen patients were enrolled. Median age was 74 (59-82) years, 8/15 (53%) were female; patients were primarily Caucasian (13/15; 87%); performance status was 0-1, with 27% (4/15) ECOG 0 and 73% (11/15) ECOG 1. This was a heavily pre-treated patient population with a median of 3 prior lines of therapy (1-6). Among patients where results were available, 79% (11/14) had abnormal FISH results including 36% with TP53 gene loss, 27% del(13q), and 18% del(11q). Sixty-seven percent (8/12) had unmutated IGHV. Of the 15 patients treated, 4 were not evaluable for efficacy due to coming off study early for toxicity (2), development of MDS unrelated to study drug (1) or death due to complications from COVID-19 infection (1). Among the 11 efficacy-evaluable patients, 82% achieved PR, and 18% had stable disease. The 12-month PFS was 43% (95%CI: 18%-66%) and the median PFS was 9.9 months (95% CI: 5.5-NA). Among the 9 responders, the median duration of response (DOR) was 5.3 months (95%CI: 1.9-NA) and 12-month DOR was 22% (95%CI:3%-51%). Five patients (33%) discontinued therapy due to adverse events, which occurred during the induction phase in 4/5 of the patients. Five patients (33%) discontinued due to progressive disease - all during the maintenance phase of treatment. Additionally, 2 patients discontinued due to MD decision; one patient developed MDS which was unrelated to duvelisib; one patient died during treatment due to complications from COVID-19. Treatment was generally well tolerated with the most common grade 3 or higher adverse events being leukocytosis (20%), alanine aminotransferase (ALT) increase (13%), aspartate aminotransferase (AST) increase (13%), oral mucositis (13%) and colitis (13%). The adverse events leading to treatment discontinuation occurred primarily during induction. The most common adverse events of any grade included fatigue in 5 patients (33%), leukocytosis, vomiting, ALT increase, AST increase, diarrhea, and elevation of alkaline phosphatase, which occurred in 4 patients each (27%).
Conclusions. We report the results of an open-label, phase 2 study utilizing a continuous dosing induction phase followed by an intermittent dosing maintenance phase of the PI3K inhibitor duvelisib in patients with relapsed/refractory CLL/SLL. Patients who received duvelisib on an intermittent schedule achieved some clinical benefit, however, the desired 12-month PFS goal of 50% was not reached. Adverse events in general were manageable and mainly related to gastrointestinal and liver toxicity, and patient died during treatment from complications from COVID-19 pneumonia. After an initial 12 weeks of continuous duvelisib treatment, the incorporation of intermittent dosing appears to be a viable option for patients with previously treated CLL/SLL.
OffLabel Disclosure:
Shouse:Kite Pharmaceuticals: Consultancy, Speakers Bureau; Beigene, Inc.: Speakers Bureau. Brown:SecuraBio: Research Funding; MEI Pharma: Research Funding; TG Therapeutics: Research Funding; Loxo/Lilly: Consultancy, Research Funding; Kite: Consultancy; iOnctura: Consultancy, Research Funding; Hutchmed: Consultancy; Grifols Worldwide Operations: Consultancy; Merck: Consultancy; Abbvie: Consultancy; BeiGene: Consultancy, Research Funding; Alloplex Biotherapeutics: Consultancy; Gilead: Research Funding; Genentech/Roche: Consultancy; Acerta/AstraZeneca: Consultancy; Pharmacyclics: Consultancy; Pfizer: Consultancy; Numab Therapeutics: Consultancy. Danilov:Astra Zeneca: Consultancy, Research Funding; Janssen: Consultancy; Cyclacel: Research Funding; MEI: Consultancy, Research Funding; Genentech: Consultancy; Bristol Meyers Squibb: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Bayer: Research Funding; Nurix: Consultancy, Research Funding; Lilly Oncology: Consultancy, Research Funding; Merck: Consultancy; GenMab: Consultancy, Research Funding.
Duvelisib is used here to treat CLL, it is the investigational agent in the trial.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal